
By Neale McDevitt
The development of antibiotic drugs was one of the 20th century’s greatest achievements, revolutionizing the way doctors care for patients by shifting their approach from a focus on diagnoses without means to intervene, to a treatment-based approach that has saved untold millions of people.
But we’ve reached a critical point in treating infectious diseases. At present, the antibiotic pipeline has slowed to a trickle. The reasons are complex but include the fact that many pharmaceutical companies have shifted toward lucrative drugs for chronic diseases and away from acute infectious disease. This, combined with the natural ability of bacteria to evolve and defend themselves against existing drugs, means that a growing number of our most potent medications are fast becoming all but useless.
How serious is this problem?
According to the Centers for Disease Control and Prevention in the United States, antibiotic-resistant infections are associated with 23,000 deaths and 2 million illnesses in the U.S. each year and more than 70 per cent of bacterial infections acquired in U.S. hospitals can resist at least one of the antibiotics commonly used to treat them.
In its first report on antibiotic resistance, tabled in April 2014, the World Health Organization classified antimicrobial resistance as a “serious threat [that] is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country.”
The Infectious Disease Society of America says “antimicrobial resistance is recognized as one of the greatest threats to human health worldwide.”
In short, the superbugs are coming and our best antibiotics are reaching their expiry date.
But if big pharma won’t save us, who will?

“It’s back in the hands of basic science,” says Samantha Gruenheid, Canada Research Chair in the Department of Microbiology and Immunology. Or, more specifically, basic scientists and thousands of undergraduate students around the globe, including 100 or so undergrads in Gruenheid’s MIMM212: Laboratory in Microbiology course.
The course is part of the Small World Initiative (SWI), a unique undergraduate research collaborative launched by Yale University in 2013, in which undergraduate students around the world look for new antibiotics in their own backyard. Literally.
“People have looked in the soil for many years for antibiotic-producing microorganisms,” explains Gruenheid. “A lot of antibiotics actually come from bacteria because they are used for bacteria vs. bacteria warfare for the environment. It’s all about competition for niches. Many antibiotics come from microbes. The classic example is penicillin, which comes from a fungus.”
Rather than rely on individual researchers working independently from one another, the idea behind SWI is to crowdsource much of the initial legwork to a global network of undergraduates to speed up the process.
Last semester, the first for Gruenheid’s class in the SWI program, she had more than 100 students collecting and analyzing soil samples. By semester’s end, they had screened almost 2,500 different bacterial isolates – the building block for potential new antibiotics – from the soil. “From that, we had them prioritize – they did tests to see if their isolates had activity against bacteria that are related to the bacteria that are drug resistant in the clinic,” says Gruenheid.
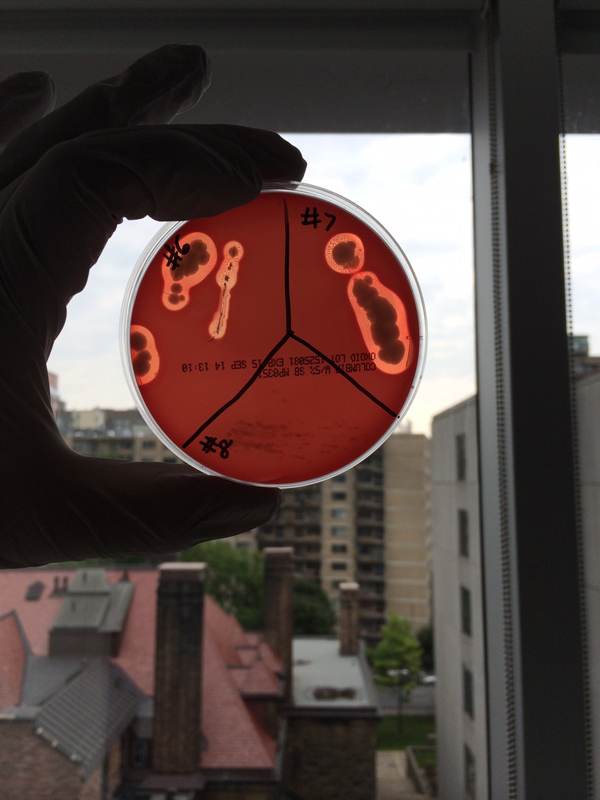
In all, Gruenheid and her class have prioritized about 100 of the most promising isolates. These samples have been frozen for further analysis. “Hopefully, we can get them far enough so that companies will be interested in developing them further,” she says.
The beauty of SWI is twofold. First, it has enlisted the help of thousands of people around the world to help looking for new antibiotics. Second, it gives undergraduate students invaluable authentic research experience in a lab setting.
“Instead of doing cookbook-style experiments – mix A and B and you should expect to see C – where you know what results to expect, students actually have a role in designing experiments and feel ownership. We even did some DNA sequencing so that the students could identify the genus of the bacteria,” says Gruenheid. “They are very excited because they feel like they are actually contributing to a real-world problem.”
“And it’s not just the students,” says Gruenheid. “As a professor, it is one of the most exciting things I’ve ever done.”
